For many years, chemists have explored the differences between liquids and solids. One difference is that liquid surfaces tend to be softer than solid surfaces (from the perspective of molecules crashing onto them). Another difference is that the surface of at least one oily liquid (perfluorinated polyether, or PFPE) actually gets stickier as it gets hotter, according to a new study by graduate student Brad Perkins and Fellow David Nesbitt. This behavior contrasts with solid surfaces, which usually get stickier when they get colder!
The discovery is part of a long-term project to study the behavior of Carbon dioxide (CO2) molecules colliding with a liquid surface in a vacuum chamber. Perkins says this research is motivated by a desire to better understand how gases enter a liquid, why gases stick to a liquid surface before they can dissolve in it, and how gases like oxygen and CO2 are transported across membranes like those in our lungs.
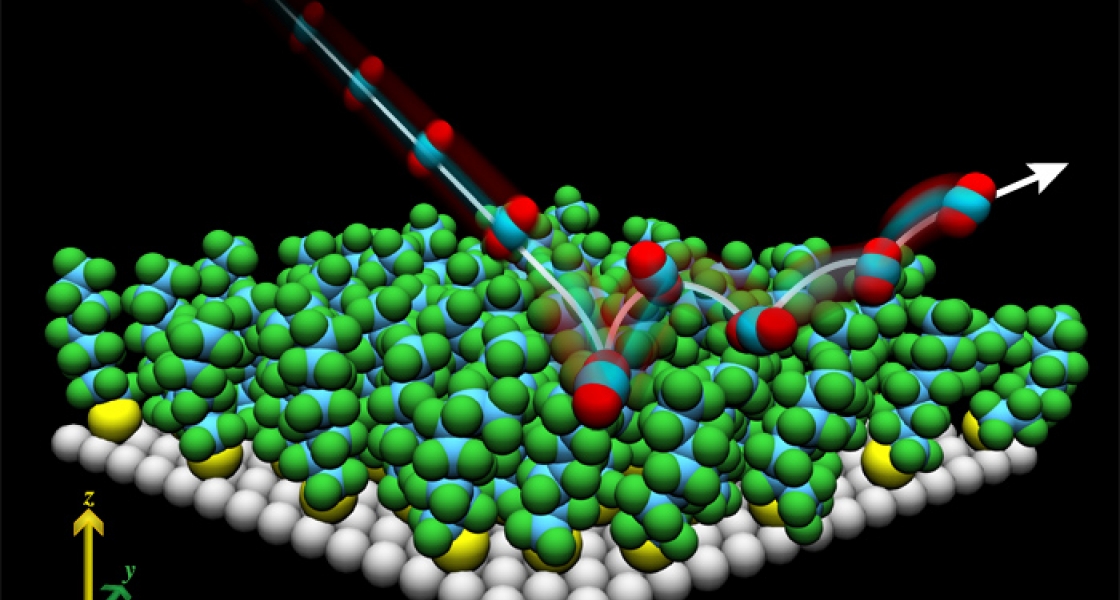
In the new study, Perkins investigated what happened when fast, cold (20 K) CO2 molecules collided with PFPE surfaces whose temperatures ranged from 232 to 323 K. As in earlier studies (see JILA Light & Matter, Fall 2005), some of the molecules splashed directly off the liquid surface, while others skipped across it, stuck around for a bit, and finally were reflected off it back into the vacuum. Interestingly, a larger fraction of the molecules stuck to the surface when surface temperatures were warmer. And, the molecules that directly splashed off the liquid surface were much hotter than expected — 600 K for the coldest liquid to 840 K for the warmest!
Apparently that warm, sticky surface really packs a punch. Recently, Perkins started watching exactly what happens when the hot CO2 molecules splash off a room temperature PFPE surface. He found that most of them cartwheel off the surface end over end, the majority tumbling forward, with just a few going backward. This is a lot like what you get bouncing a tennis ball off a racket at a glancing angle, which both rotationally excites it and gives it “top spin.” A few CO2 molecules even lift off like a helicopter, spinning parallel to the surface. Another small fraction exits in a corkscrew pattern.
In contrast, the molecules that spent a while sticking to the surface before hopping off into the vacuum behaved much more uniformly. They left the surface at the same temperature as the PFPE and showed no particular preference for a specific pattern or direction of departure.
One curious finding was that only the rotation and translation of the cold incident CO2 molecules were excited, i.e., heated up, when they splashed off of or stuck to the surface. In both cases, the vibrations in the CO2 “remembered” where they came from and remained cold throughout the entire interaction with the surface. This result likely reflects the short amount of time (a few picoseconds) that the CO2 spends on the surface, regardless of exactly what happens there. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.